Boyle's Law Worksheet Answers 1.00 L Of A Gas
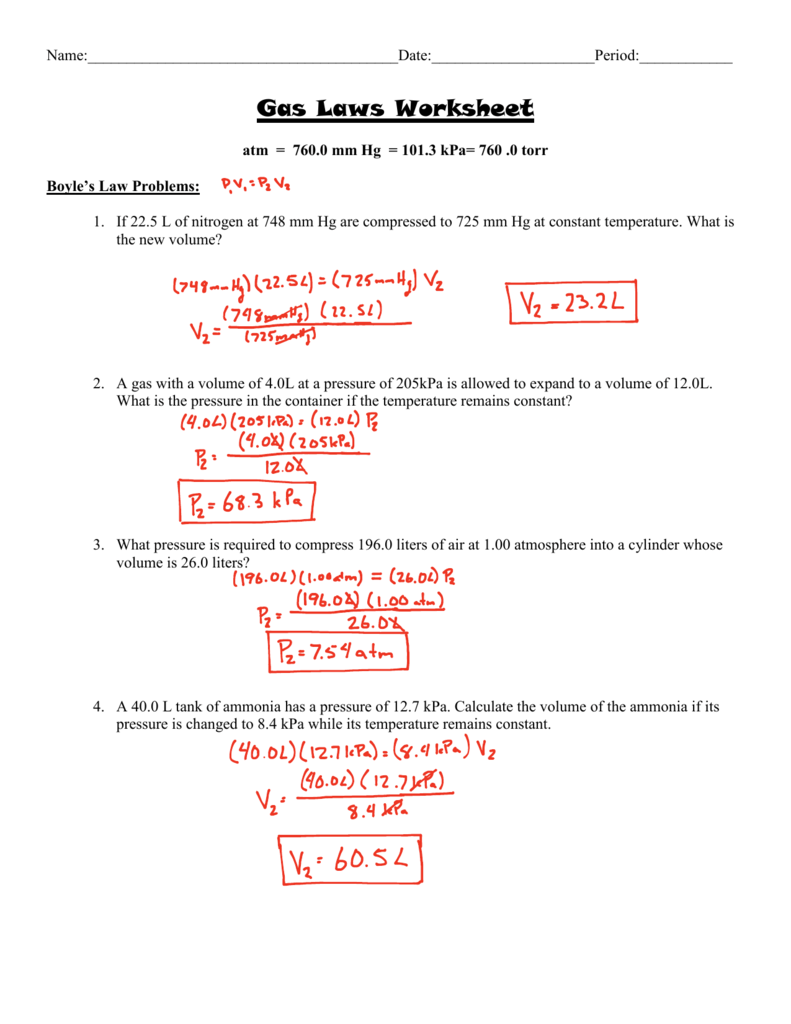
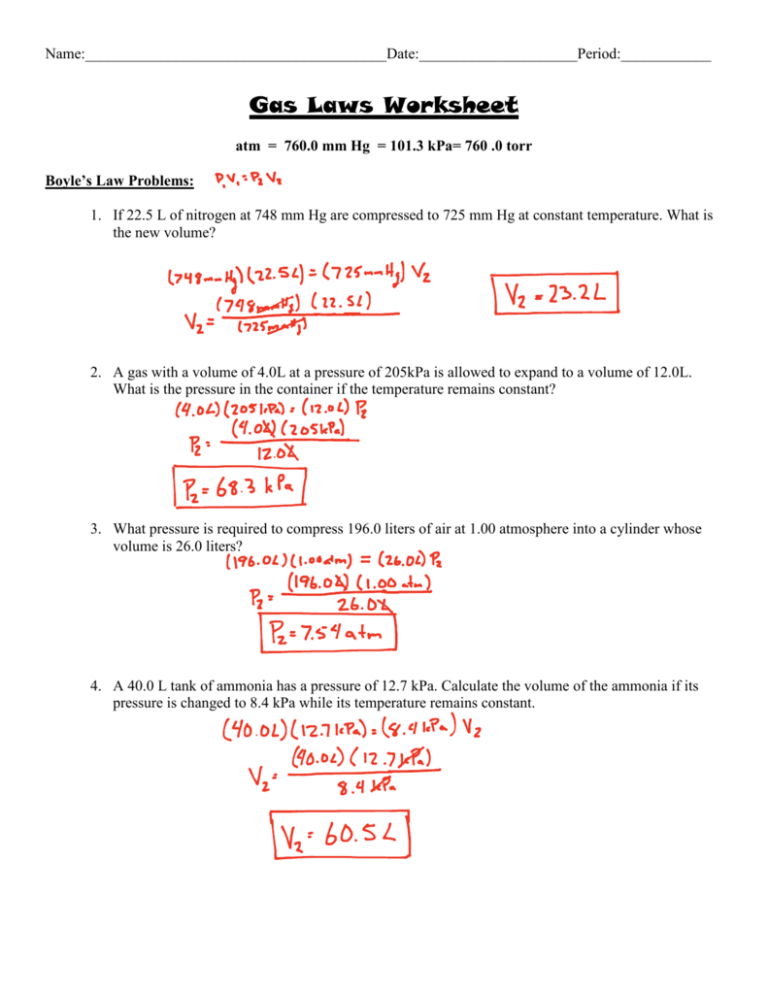
Gas laws worksheet atm = 760.0 mm hg = 101.3 kpa= 760.0 torr boyle’s law problems: Use boyle's law to answer the following questions:

Boston Worksheet — Boyle And Charles Law Worksheet Answers
Boyle, charles, and combined gas laws author:

Boyle's law worksheet answers 1.00 l of a gas. Homework 1 on boyles’ law use boyles’ law to answer the following questions: Calculate the new volume of the balloon. What is the new pressure of the gas?
Gas law packet answers 1. 1) 1.00 l of a gas at standard temperature and pressure is compressed to 473 ml. (740.0 mmhg) (2.00 l) =(760.0 mmhg) (x) problems:
1) 1.00 l of a gas at standard temperature and pressure is compressed to 473 ml. What is the final pressure in the two bulbs, the temperature being constant and the same in both bulbs? What is the volume when the pressure is increased to 60.0 mm hg?
Calculate the decrease in temperature when 2.00 l at 20.0 °c is compressed to 1.00 l. A gas occupies 1.00 l at standard temperature. A balloon that contains 1.50 l of air at 1.00 atm is taken underwater to a depth at which the pressure is 3.00 atm.
Each of these laws can be derived from this law. What is the volume of this gas at 127.0 °c? _____ gas law practice use boyle’s law to answer the following questions:
2) in a thermonuclear device, the pressure of 0.050 liters of gas within the bomb casing reaches 4.0 x. The valve between the 2.00 l bulb, in which the gas pressure is 1.00 atm, and the 3.00 l bulb, in which the gas pressure is 1.50 atm, is opened. If 22.5 l of nitrogen at 748 mm hg are compressed to 725 mm hg at constant temperature.
2) a gas exerts 1.15 atrrr of pressure in 800 mm3 container. A gas with a volume of 4.0l at a pressure of 205kpa is allowed to expand to a volume of 12.0l. Boyle's and charles' laws worksheet.
If we took 2.00 liters of gas at 1.00 atm and compressed it to a pressure of 6. The correct answer is given in parentheses at the end of the problem. When the bomb casing is destroyed by the explosion, the gas is.
Boyles’ law worksheet 1) 1.00 l of a gas at standard temperature and pressure is compressed to 473 ml. When the bomb casing is destroyed by the explosion, the gas is. 1) 1.00 l of a gas at standard temperature and pressure is compressed to 473 ml.
First of all, 2.20 l is the wrong answer. Use boyles’ law to answer the following questions: 2) in a thermonuclear device, the pressure of 0.050 liters of gas within the bomb casing reaches 4.0 x 106 atm.
Use boyles’ law to answer the following questions: Xv 1.00 atm = 760 mmhg 1.00 atm = 101300 pa 1.00 atm = 760 torr 1.00 atm = 101.3 kpa 1.00 atm = 14.7 psi example a gas occupies a volume of 5 4 l at a pressure of 1.06 atm. What is the new pressure of the gas?
What is the volume at 60.0 °c? Show which values you have, which values. 1) 1.00 l of a gas at standard temperature and pressure is compressed to 473 ml.
If the balloon is squeezed into a 0.500 1.0 l of a gas at standard temperature and pressure is compressed to 473 ml. A gas occupies 900.0 ml at a temperature of 27.0 °c.
A gas has a pressure of 0.470 atm at 60.0 °c. Boyle’s law the volume (v) of an ideal gas varies inversely with the applied pressure (p) when the temperature (t) and the number of moles (n) of the gas are constant. What is the new pressure of the gas?
A gas occupies 12.3 liters at a pressure of 40.0 mm hg. 2) in a thermonuclear device, the pressure of 0.050 liters of gas within the bomb casing reaches 4.0 x 106 atm. V 1 p 1 = v 2 p 2.
1) 1.00 l of a gas at standard temperature and pressure is compressed to 473 ml. Gas law worksheet pv = nrt. 2.11 atm 2) in a thermonuclear device, the pressure of 0.050 liters of gas within the bomb casing reaches 4.0 x 106 atm.
Use boyles’ law to answer the following questions: View gas_law_practice_worksheet_2020.docx from science 101 at camino nuevo high school. 600.0 ml of air is at 20.0 °c.
What is the new pressure of the gas? 1.00 l of a gas at standard. What is the new pressure of the gas?
Convert 1.5 atm to kpa latm=101.325 kpl. You are told that, initially, the pressure in the container is 765 mm hg and the volume is 1.00 l. 1.00 atm = 760.0 mm hg = 76 cm hg =101.325 kpa = 101, 325 pa = 29.9 in hg k = °c + 273 fo =1.8co +32 co = fo −32 1.8
Then plug into the equation and solve for x, like this: What is the volume at. 9) a 1.00 l balloon is filled with helium at a pressure of 1.20 atm.
1) p 1 v 1 = p 2 v 2 twice (1.00 atm) (2.00 l) = (x) (5.00 l) x = 0. Hospitals buy 400 l cylinders of oxygen gas compressed at 150 atm pressure. What volume (v2) of oxygen can a cylinder supply at this lower pressure?
Under what conditions do gases deviate from ideal behavior? If a diver has 0.050 l of gas in his blood under a pressure of 250 atm, then rises instantaneously to a depth where his blood has a pressure of 50.0 atm, what will the volume of gas in his blood be? 2) in a thermonuclear device, the pressure of 0.050 liters of gas within the bomb casing reaches 4.0 x 106 atm.
13.2 boyle's law worksheet answers. 2.11 atm 2) in a thermonuclear device, the pressure of 0.050 liters of gas within the bomb casing reaches 4.0 x 106 atm. 8) divers get “the bends” if they come up too fast because gas in their blood expands, forming bubbles in their blood.
If a gas at 25.0 °c occupies 3.60 liters at a pressure of 1.00 atm, what will be its volume at a pressure of 2.50 atm? Boyles’ law use boyles’ law to answer the following questions: Assume the temperature remains constant.
Convert 50.0°c to 323 k and 25.0°c to 298 k. Determine the pressure change when a constant volume of gas at 1.00 atm is heated from 30.0 °c to 40.0 °c. A gas occupies 1.56 l at 1.00 atm.
What is the new volume? What is the new pressure of the gas? An ideal gas occupies 400ml at 270 mm hg and 65°c.
A as ple contained in a cylinder equipped with a moveable piston occupie 00.0 at a pressure

5.2 The Simple Gas Laws I Boyle's Law

Ideal Gas Law Gizmo Answers + My PDF Collection 2021
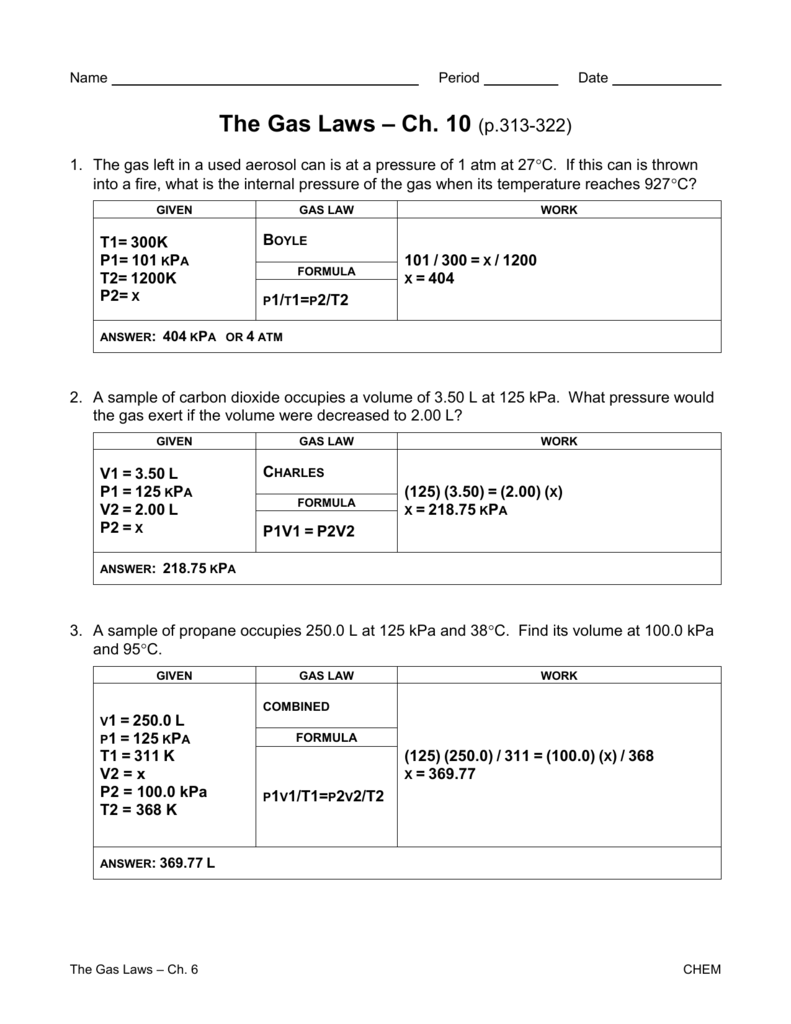
Gas Laws Worksheet churchillcollegebiblio
32 Ideal Gas Law Worksheet Answer Key Free Worksheet

Boyles Law Worksheet Answer Key Worksheet List
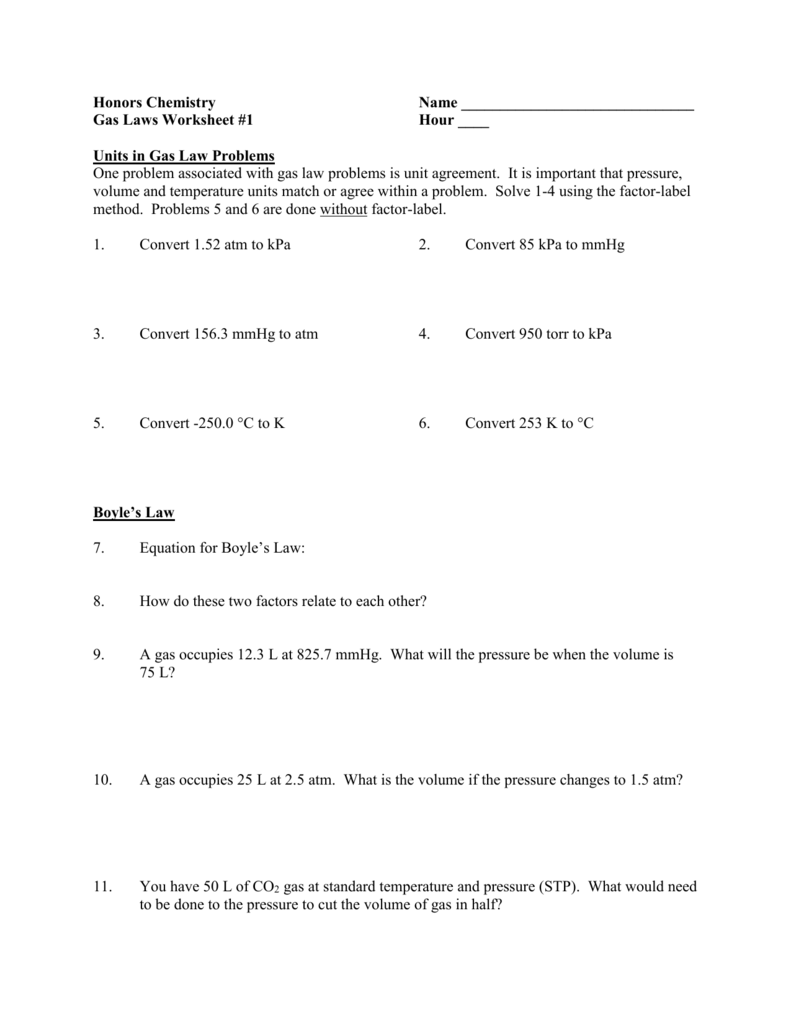
Chemistry Gas Laws Worksheet Answers / Ideal Gas Law

The Ideal Gas Law Worksheet Answer Key — Villardigital

Charles law example problems with answer Canada

Key Charles Law Worksheet Answers kidsworksheetfun

Gas Laws Review Part 1 Boyles, Charles, Gay

Chemistry 60 Finding Volume Of Gas Worksheet Pdf

Ideal Gas Law Practice Worksheet Answers bradfieldschool

Ideal Gas Law Practice Worksheet Answers bradfieldschool